
Breakthrough imaging technology shows embryo implantation for the first time
The beginning of life inside the womb has always carried an air of mystery. For decades, scientists have studied embryos under microscopes and examined fragments of the implantation process through snapshots.
Yet, one of the most critical stages – the moment an embryo embeds itself into the uterus – remained hidden. This step decides whether pregnancy will succeed or fail, yet no one had ever witnessed it in full motion.
That has now changed. Researchers at the Institute for Bioengineering of Catalonia (IBEC), working with Dexeus University Hospital, have captured real-time 3D images of a human embryo implanting.
This marks the first time the process has been recorded in such detail, offering answers to questions that have puzzled scientists for decades. Their findings appear in the journal Science Advances.
Live view of embryo implantation
Implantation failure is one of the leading causes of infertility. It accounts for about 60 percent of spontaneous miscarriages. Until now, the process in humans could not be observed live. Researchers only had access to scattered still images.
Samuel Ojosnegros is the principal investigator of IBEC’s Bioengineering for Reproductive Health group and leader of the study.
“We have observed that human embryos burrow into the uterus, exerting considerable force during the process,” he said. “These forces are necessary because the embryos must be able to invade the uterine tissue, becoming completely integrated with it.”
“It is a surprisingly invasive process. Although it is known that many women experience abdominal pain and slight bleeding during implantation, the process itself had never been observed before.”
Embryo reshapes uterine matrix
To advance, the embryo does more than rely on enzymes that break down tissue. It must also push physically against the collagen-rich uterine matrix. Collagen, the same protein that strengthens tendons and cartilage, forms a stiff barrier.
“The embryo opens a path through this structure and begins to form specialized tissues that connect to the mother’s blood vessels in order to feed,” said Ojosnegros.
The study revealed that embryos actively remodel their environment.
“We observed that the embryo pulls on the uterine matrix, moving and reorganizing it. It also reacts to external force cues,” said Amélie Godeau, a researcher in the Ojosnegros group and co-first author of the study. “We hypothesize that contractions occurring in vivo may influence embryo implantation.”
Effective implantation requires both chemical and mechanical strategies. The embryo generates pushing and pulling forces to reorganize tissue, creating the conditions needed for blood vessel connection.
Mechanical forces and embryo behavior
The research highlights how physical cues shape early development. The embryo’s forces are not random but regulated and adaptive.
According to the study, human embryos display dynamic traction patterns, adjusting their grip as they move deeper.
This differs significantly from other species. For instance, in mice the uterus plays an active role, folding around the embryo. Human embryos instead behave more aggressively, penetrating tissues with sustained force.
The difference suggests species-specific implantation strategies evolved to match reproductive environments.
These findings also reveal that embryos interact with the extracellular matrix in ways similar to invasive cancer cells.
Both systems remodel their surroundings, though embryos do so under tightly regulated conditions that lead to healthy growth rather than disease.
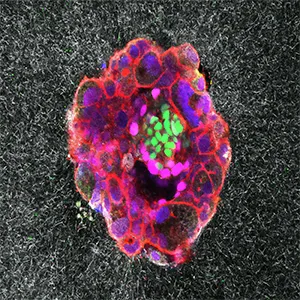
Implications for fertility treatments
This work could influence assisted reproduction practices. A clearer picture of implantation may improve embryo selection and boost pregnancy success rates. It also sheds light on why some embryos fail to attach or develop properly.
The ability to analyze implantation in detail may shorten conception times in clinical settings.
Better insights into embryo mechanics and tissue interactions might also help refine fertility treatments for patients struggling with repeated failures.
Additionally, the study suggests that monitoring embryo force generation may become a future diagnostic tool.
Measuring how embryos push and pull could help identify those with greater implantation potential.
Womb recreated in lab
To capture these details, the IBEC team designed a platform that mimics the uterine environment. It allowed embryos to implant outside the uterus under carefully controlled conditions.
Using a gel made of artificial collagen and essential proteins, they recreated the uterine tissue structure.
This platform enabled real-time fluorescence imaging, revealing how embryos generate force and interact with their surroundings. Both human and mouse embryos were studied to highlight species differences.
Mouse embryos rely on the uterus folding around them, creating a crypt. Human embryos, however, drive themselves inward, penetrating tissues and expanding from within.
“Our platform has enabled us to quantify the dynamics of embryo implantation and determine the mechanical footprint of the forces used in this complex process in real time,” said Anna Seriola, IBEC researcher and co-first author of the study.
The study is published in the journal Science Advances.
—–
Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Check us out on EarthSnap, a free app brought to you by Eric Ralls and Earth.com.
—–












