
Scientists have changed their minds again about the composition of Earth's core
Far beneath our feet, beyond the Earth’s rocky mantle and molten outer core, lies a dense, solid sphere of iron and other elements like carbon.
This inner core is Earth’s deepest secret, shaping the magnetic field that shields our planet. Yet, scientists still puzzle over how it first formed and what ingredients allowed it to crystallize.
A new study by researchers from the University of Leeds, University of Oxford, and University College London provides fresh insight.
Their findings reveal that carbon may have been the key element that enabled the inner core to solidify billions of years ago.
Why Earth’s core composition matters
The makeup of Earth’s core determines not only its density but also its thermal and magnetic behavior.
Seismology shows that the core is less dense than pure iron, meaning lighter elements must be present. Candidates like silicon, sulphur, oxygen, and carbon have all been considered.
The latest simulations show that carbon, in particular, may have been essential. Without it, the inner core’s crystallization would have required impossible conditions, making its very existence a mystery.
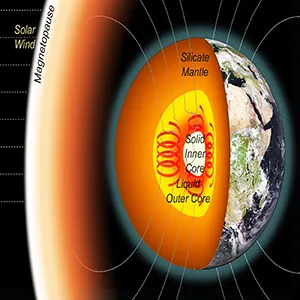
The supercooling paradox
Liquids rarely freeze solid the instant they cool past their melting point. Instead, they must be supercooled, chilled further before crystals can form.
For molten iron alone, estimates suggested that a massive 800 to 1000 °C (1472 to 1832 °F) of supercooling would be needed.
If that had occurred, the consequences for our planet would have been dramatic.
The inner core would have grown far too quickly, disrupting the delicate balance of heat flow inside Earth and destabilizing the magnetic field that protects life from harmful solar and cosmic radiation.
Scientists know this did not happen. Geophysical evidence points instead to a modest level of supercooling, limited to about 250 °C (482 °F).
That small difference between theoretical predictions and observational reality has puzzled researchers for decades.
This contradiction, known as the inner core nucleation paradox, continues to inspire debate, experiments, and advanced simulations aimed at uncovering the true chemistry of Earth’s deepest layers.
Simulating the impossible
To resolve the paradox, the research team ran atomic-scale simulations of iron mixed with different light elements.
They tested silicon, sulfur, oxygen, and carbon, and watched how each affected the first step of freezing: nucleation.
“Each of these elements exists in the overlying mantle and could therefore have been dissolved into the core during Earth’s history,” explained co-author Associate Professor Andrew Walker from the University of Oxford.
“As a result, these could explain why we have a solid inner core with relatively little supercooling at this depth. The presence of one or more of these elements could also rationalize why the core is less dense than pure iron, a key observation from seismology.”
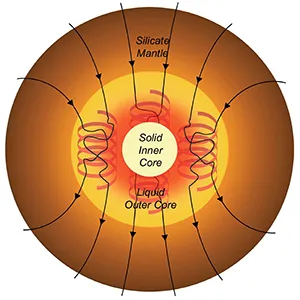
Carbon drives Earth’s core freezing
The simulations revealed that silicon and sulfur actually hindered freezing, which would have increased the supercooling requirement. Carbon, however, had the opposite effect.
With 2.4 % carbon, the required supercooling dropped to about 420 °C (788 °F). Still too high, but much closer to reality.
Extrapolating further, a carbon content of 3.8 % reduced the figure to 266 °C (511 °F), which is consistent with geological evidence.
This makes carbon the only element so far shown to both enable nucleation and align with seismic observations.
No seeds needed
Unlike hail forming around dust in clouds, Earth’s core appears to have frozen without “nucleation seeds.”
Tests of possible seed materials, like oxides or metals, show they would melt or dissolve before reaching the center. That leaves chemistry itself, specifically the presence of carbon, as the driver of solidification.
“It is exciting to see how atomic scale processes control the fundamental structure and dynamics of our planet,” said lead author Dr. Alfred Wilson from the University of Leeds.
“By studying how Earth’s inner core formed, we are not just learning about our planet’s past. We’re getting a rare glimpse into the chemistry of a region we can never hope to reach directly and learning about how it could change in the future.”
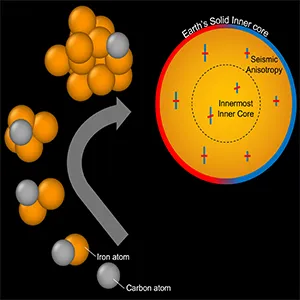
Carbon’s role in Earth’s core
The research also helps refine estimates of when the inner core formed. Some argue it began crystallizing over two billion years ago, while others suggest it happened less than half a billion years ago.
By identifying carbon as the critical player, scientists can narrow down these timelines.
This enables a better understanding of how Earth’s deep engine has evolved to influence the magnetic field, surface conditions, and even the long-term habitability of our planet.
The message is clear: carbon is more than a surface element of life. It may also be the hidden architect of Earth’s inner heart, shaping its stability across geological time.
The study is published in the journal Nature Communications.
—–
Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Check us out on EarthSnap, a free app brought to you by Eric Ralls and Earth.com.
—–













