
Introducing 'goldene': the world's newest supermaterial that is one atom thick
Gold behaves differently when it is reduced to a single layer of atoms. Electrons take new routes and light couples to the surface in surprising ways. A team reports they have isolated such a layer and given it a fitting name: “goldene,” echoing graphene’s single-atom carbon sheet.
Why would anyone fuss over atom-thick gold? Shrinking to two dimensions can unlock properties you don’t see in bulk metal.
That includes tunable electrical behavior, stronger interactions with light, and a hyperactive surface useful for catalytic reactions.
This new work shows how to make a freestanding gold sheet just one atom thick, rather than only growing ultrathin films on a supporting surface.
How they made goldene
According to Lars Hultman, a materials scientist at Linköping University in Sweden and senior author of a study in Nature Synthesis, the method could “expand the boundaries of what it’s possible to do with materials,” and it starts with a naturally layered crystal.
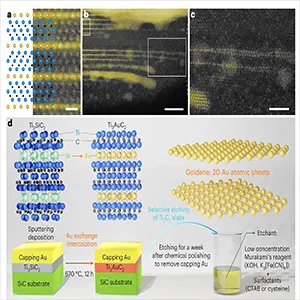
The group first created a gold-containing “MAX phase,” a crystal that stacks atomic layers in a repeating pattern.
They began with Ti₃SiC₂, then heated a gold-coated film to approximately 670°C (1,238°F), allowing gold atoms to replace silicon and form Ti₃AuC₂.
In earlier work, that swap left atom-thick gold trapped between other layers.
“The good news was that we had gold layers that were just one atom thick,” Hultman says. “The bad news was that they were stuck inside the host crystal.”
Freeing the monolayer
Metals prefer to bead up, not spread flat at the atomic scale. The team avoided that by etching away the non-gold layers while protecting the newly exposed gold.
They used Murakami’s reagent, a classic etchant that reacts strongly with titanium and carbon but largely ignores gold under the right conditions.
Surfactants acted like traffic control on the gold surface. CTAB, a bulky molecule, and small sulfur-bearing molecules such as cysteine or cysteamine attached to gold and helped keep the sheet from curling or merging into blobs during the etch.
Tuning goldene’s chemistry
Selectivity mattered. If the etchant was too strong, the structure broke into nanoparticles. If it was too weak, the process dragged out and damaged the sheet.
The sweet spot used low concentrations paired with the right surfactants. The etch ran in darkness to prevent light-driven reactions that generate cyanide species capable of dissolving gold.
Spacing between the gold layers in the starting crystal mattered too.
Layers that were too close attracted each other as they were freed, merging into thicker pieces. More separation gave surfactants time to slip in and stabilize the sheet.
Tighter atomic spacing
Electron microscopes revealed true monolayers: freestanding gold sheets only one atom thick. The flakes were not large; most spanned from a few nanometers to about 100 nanometers across (about 0.0000039 inches).
As with many single-atom materials, the sheets showed natural ripples and slightly curled edges, but they remained single layers rather than stacks.
Measurements showed the distance between neighboring gold atoms shrank by about 9% compared with ordinary gold.
Confinement to two dimensions can tighten bonding, and that’s reflected in the shorter spacing. Elemental mapping confirmed the identity: the sheets were truly gold, not a titanium-based lookalike.
What the x-rays revealed
X-ray photoelectron spectroscopy found a higher binding energy shift – about 0.88 electronvolts – compared with regular metallic gold.
That shift signals a different electronic environment for goldene’s atoms, consistent with a two-dimensional metal rather than bulk gold.
Atomistic simulations suggest that a perfect goldene sheet remains stable at room temperature. The same modeling helps explain the observed ripples and the tendency for edges to curl or aggregate when surface chemistry is not tuned correctly.
That guidance, together with the experiments, points to practical levers – etch strength, surfactant choice, light exposure, and layer spacing – to improve yield and size.
Why goldene matters
Gold already plays a role in electronics, photonics, sensing, and medicine. Flattening it to a single layer boosts surface area and tweaks electron behavior.
That combination could help catalysts that convert waste streams into useful chemicals, enhance light-harvesting in solar components, or make photothermal therapies more efficient by heating tumors locally with less material.
Because goldene exposes so many atoms directly on the surface, you can get more effect from less metal than with larger particles. That can cut costs while trimming environmental impact tied to mining and refining.
In short, the team hid a monolayer of gold inside a layered crystal, then etched away everything else while “soaping” the gold so it stayed flat.
The result is a freestanding, one-atom-thick metal sheet with clear structural and electronic signatures that differ from those of bulk gold – and a wide-open set of possibilities that now moves from wish list to working material.
The full study was published in the journal Nature Synthesis.
—–
Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Check us out on EarthSnap, a free app brought to you by Eric Ralls and Earth.com.
—–













